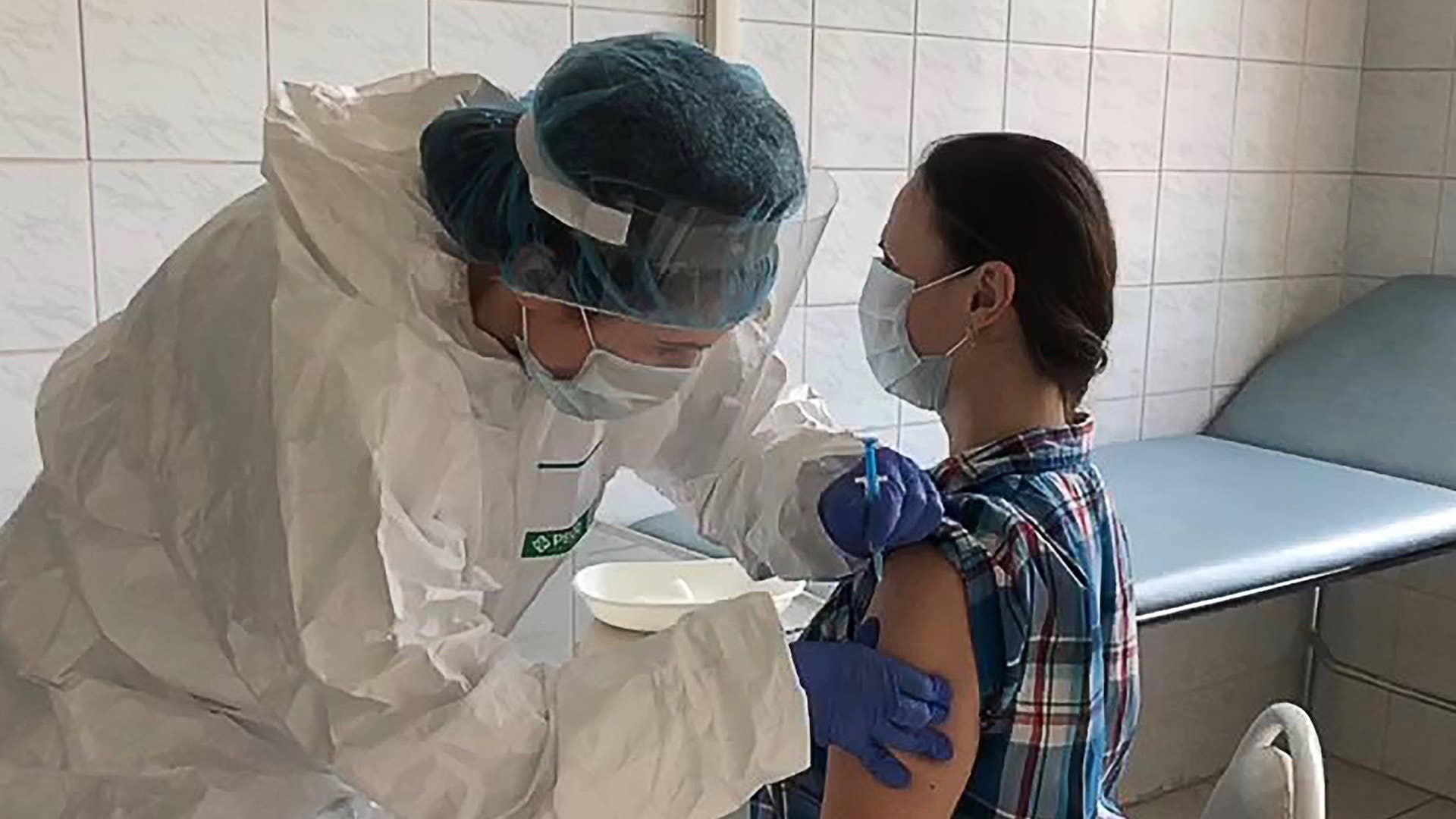
The race to an effective coronavirus vaccine may soon be over. According to CNN, Russia is looking to become the first country to approve a vaccine for public use sometime before Aug. 10. The outlet notes the country has yet to provide any data from its clinical trials; the lack of transparency has, of course, raised many questions about the experimental vaccine's efficacy and safety.
Russia's sovereign wealth fund is financing the ongoing coronavirus trials that began back in June. The vaccine in question was developed by the Moscow-based Gamaleya National Research Center for Epidemiology and Microbiology. Russian officials said once the vaccine is approved, the country's frontline healthcare workers will be the first to receive it.
The country will reportedly complete phase 2 of its clinical trials by Aug. 3. The third phase will take place at the same time health care workers begin receiving the vaccination.
The head of Russia's sovereign wealth fund Kirill Dmitriev called the speedy development "a Sputnik moment," a reference to the Soviet Union's successful launch of the world's first artificial satellite.
"Americans were surprised when they heard Sputnik's beeping," he told CNN. "It's the same with this vaccine. Russia will have got there first."
Moderna Inc. announced Monday it has started phase 3 of its COVID-19 clinical trials, which are being backed by the U.S. government. The third and final phase is expected to enroll 30,000 adult volunteers at nearly 90 sites across the country. Moderna heads said they hope their vaccine gets FDA approval by the end of the year or early 2021.
"Having a safe and effective vaccine distributed by the end of 2020 is a stretch goal, but it’s the right goal for the American people," said Dr. Francis S. Collins, the head of the National Institutes of Health. "The launch of this Phase 3 trial in record time while maintaining the most stringent safety measures demonstrates American ingenuity at its best and what can be done when stakeholders come together with unassailable objectivity toward a common goal."